Absolutely Hot
Look closer at a common chemical reaction to understand heat and energy and learn how they are measured.
Teacher Tips!
Many activities have a teacher view and a student view, and teachers can switch between those views by clicking the blue button in the upper-right. Students will not see this option - only teacher accounts see both views. The teacher view will start with overview text, if available, to frame the activity and get you started. This view will also have teacher tips and suggested answers to student questions spread throughout the activity. The teacher text interleaved with student-facing text will be in italics and should appear as a different color on your screen. Teacher tips are designed to help you deliver a learning experience that is best suited for your classroom.
Printing Reminder
Whichever view you see on your screen is what will print. You can print this activity without teacher tips by selecting the student view, or print with teacher tips by switching to teacher view. Simply use the standard print function available for your web browser. No extra steps are required.
Title of Activity: Absolutely Hot
Brief Description: In this activity, students look at an exothermic reaction to understand the mole as a standard for concentration and the Kelvin as a unit of temperature.
Target Grade Level: Grades 8-12
Discipline or Course: Physical Science
Estimated Time Required: One 45-minute session
Individual / Partner / Group Work: Teams of 3-4
Key Vocabulary:
- Mole
- Kelvin
- Boltzmann’s constant
Teacher Prep:
Ice should be crushed or shaped in very small cubes to establish Triple Point. Be conscious of disposal of the plaster solution as it will harden in the pipes.
Differentiation:
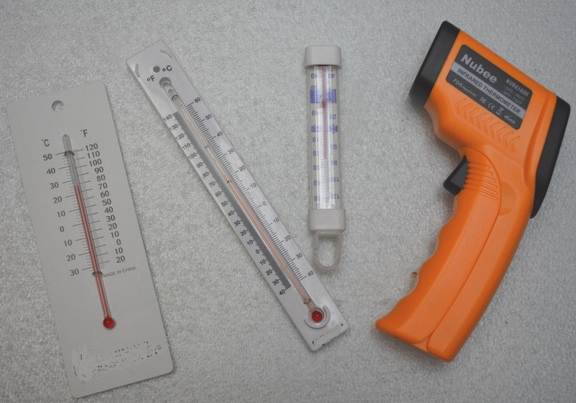
A graphic of a thermometer with both Celsius and Kelvin can help students understand that the measurements are parallel but extensive explanation and discussion may be needed to clarify the difference between a direct value (Kelvin) and a comparative measurement (Celsius.)
Taking It Further:
Variation can be determined in almost any product; this makes the procedure ideal for home individualization.
Suggestions for Remote Learning:
There are many online programs which enable students to combine their own data to create a larger database. This improves the activity. At-home investigators should make an effort to contact others to increase the database.
STUDENT CONTENT BELOW
Background Knowledge and Extensions:
The traditional measurement of temperature looked at the effect of energy on matter, a relative measurement: “This is as warm as…“ The temperature was expressed in degrees Fahrenheit or Celsius. By contrast, a Kelvin is an absolute unit. Its magnitude is set by fixing the numerical value of the Boltzmann constant equal to exactly 1.380649 × 10-23 joules per kelvin. A change of one kelvin is the same amount of temperature change as one degree Celsius, but the Kelvin scale is “absolute” in the sense that it starts at absolute zero, or what Kelvin and other scientists called “infinite cold.” (0 K = -273.15 degrees C = -459.67 degrees F. Room temperature is about 70 degrees F, 21 degrees C or 294 K.) The absolute, thermodynamic temperature of an object provides information on how much average energy of motion (kinetic energy) its atoms and molecules have.
Many processes—from chemical reactions to observations of distant galaxies—depend upon accurately understanding the temperature of the phenomenon. That requires precision in the tool and in the way we use it. In this activity, you’ll compare the precision of several instruments to measure temperature. You’ll use Celsius rather than Kelvin but will consider how an absolute unit might make a difference.
The activity begins with the calibration of a thermometer in the traditional way, using ice water as a sort of artifact. You will review the concept of triple point, the temperature where ice, water, and vapor can co-exist. Then you will monitor the exothermic reaction when the plaster of Paris is hydrated. In chemistry, a hydrated compound is a compound with one or more water molecules bound to each formula unit. In this reaction, energy is released as the larger, more stable molecule is formed. This energy is reflected as a change in the temperature of the solution. The concentrations in the directions produce a very small change in temperature. Very soon after that change is noted, the difference between the solution and ambient temperature causes the temperature to change again.
The constant which is used for temperature is The Boltzmann constant (kB) which relates temperature to energy. It’s named for Austrian physicist Ludwig Boltzmann (1844–1906), who is considered a pioneer in the field of statistical mechanics.
Concentration is defined by Avogadro’s number and the mole. In the process of redefining the kilogram, two competing models were investigated. Some metrologists favored using the mole as a unit of mass, and creation of a perfect mole sphere of silicon. Ultimately, the use of Planck’s Constant H earned the support of the majority of metrologists because it also simplified the establishment of standards for voltage and current.
Introduction
Explore measurement and measuring instruments for temperature, using an absolute standard rather than a comparative measurement. Kelvin may seem similar to Celsius degrees on the surface, but the difference is absolute.
Plaster of Paris is used to make casts for broken bones. The hydrated chemical is applied to bandages that are wrapped around the bone. The plaster of Paris then hardens. Here’s an example of where the energy in a plaster of Paris reaction might be very important. Veterinarians who work with wild animals sometimes have to make casts for them. These “patients” are not used to people and have to be sedated for a brief time. Imagine a group of veterinarians working to find a quick-setting plaster of Paris procedure that will allow them to set a cast that hardens as quickly as possible. One problem is that because the reaction is exothermic, the cast can burn the animal if the plaster of Paris is too hot. The maximum temperature varies depending on the species of animal. In order to balance speed with safety, veterinarians need a precise thermometer. The group of veterinarians has asked you to determine which of the four brands of thermometers is the most precise. You will evaluate the thermometers using the same chemical reaction the veterinarians use to make plaster of Paris to ensure that the results are applicable.
To make solutions that are reproducible, you will use measurements in grams that are then converted to moles. A mole is a unit of measurement that describes a specific number of particles. Using a unit that refers to a number is not such a strange concept if you think about it. Have you ever gotten a dozen ears of corn? The word dozen describes how many ears of corn you have, which is the number 12. In the same way, the mole stands for 6.02214076 × 1023 particles. The particles measured with the mole include atoms, molecules, ions, groups of ions described by the formula of an ionic compound, and electrons.
The mole was recently redefined in 2018. The mole was used to equal the number of atoms experimentally found in 12 grams of carbon-12. The mole is also called Avogadro’s number to honor the Italian physicist Amedeo Avogadro (1776–1856). The molar mass is the mass of 1 mole of particles. Because different particles have different masses, their molar masses are different. For carbon and for all other elements, the molar mass is the same numerical value as the atomic mass of one atom. The only difference is in the units: molar mass is measured in grams per mole (g/mol), whereas atomic mass is measured in atomic mass units (AMU). Molar mass allows you to easily convert from the number of atoms/molecules to the number of moles to the number of grams in a chemical equation. For example, in a chemical equation, coefficients can refer to atoms/ions/molecules or to moles. Using molar mass, moles can be converted to grams.
Materials (Per group)
Procedure I:
- 100 ml beakers
- Stirring rods
- Ice and water slurry (The ice should be in small chips)
- Several thermometers with range ~freezing point.
- (Thermometers with a range near 100oC are optional)
Procedure II:
- 36.2 g plaster of Paris
- 4 different temperature tools with range 30-40o Celsius
- Water ~35o Celsius
- Balance
- Styrofoam cup
- Small plastic bag (baggie without zip lock or one you might use for dog waste pickup)
Safety Notes:
Use eye protection when using chemicals and when chipping ice.
Experiment:
Procedure I:
Explore the accuracy of at least one of the thermometers available to you.
- Create a beaker of ice and water slurry. Let the ice water sit for at least 5 minutes.
- Record the temperature of the slurry with all of the thermometers available to you that record a range of ~0oC. Which of the thermometers was most accurate? If the thermometer is correct it should read 0oC. Make sure that students have let their cups sit for a few minutes before measuring.
- If you have thermometers with a range of ~0oC record the temperature of the boiling water with all of the thermometers available. Do not try this unless the thermometer has a high-temperature capacity. The thermometer will be destroyed. Which of the thermometers was most accurate?
Procedure II:
- Prepare a beaker with 100 ml of water at about 35oC.
- From a collection of thermometers provided, select 3 that you think are most appropriate to measure slight changes in your water solution, (Check the temperature range.)
- Measure out the plaster of Paris. (Don’t forget to tare the filter paper.)
- Slowly dissolve the plaster of Paris in the solution. Once it is all in solution, record the change of temperature every minute for 10 minutes. (Do not stir with your thermometers.)
- Graph the change in temperature over time for each of the thermometers.
- Carefully rinse the thermometers and dispose of the plaster/water solution outside (not in a drain).
Analysis:
- How many significant figures can your balance produce? (How precise is the balance?) This will vary. It should represent one level beyond the most fine marks on the balance if the readout is analog.
- Which of the thermometers provided gives the most precise measurements?
- Were some of the thermometers unable to record the difference in temperature?
- What caused the initial change in temperature? The reaction to hydrate the plaster of Paris is exothermic.
- What caused the later change in temperature? The energy is dissipated into the air.
- One mole of plaster of Paris in solution is 145.15 grams. What is the molarity of the solution that you created? (Remember a one molar solution is one mole in 1 liter of water.) 0.025
- How much did the temperature increase in Kelvin? Approximately 2 degrees.
- How is a Kelvin degree different from a Celsius degree? It is an absolute measure of energy.
Conclusion:
What did you learn?
What else would you like to know?